Follow Us x
Testimonials
★★★★★
Chris and Tori are absolutely amazing! Every time I walk in I am welcomed with happy friendly faces. They are knowledgeable and listen to my expectations. Highly recommend!
Chris and Tori are absolutely amazing! Every time I walk in I am welcomed with happy friendly faces. They are knowledgeable and listen to my expectations. Highly recommend!
Kellie R. | SCARBOROUGH, ME
★★★★★
Being a baby boomer, I worshipped the sun and slathered up with Baby Oil and iodine. At seventy years old, I am paying for every minute I bathed in the sun. Maine Laser Skin Care has helped me literally turn back the hands of time! They are so adept with Botox and fillers but also stay cutting edge treatments such as RF micro-needling,a procedure that has rejuvenated my skin beyond my expectations. Like
Being a baby boomer, I worshipped the sun and slathered up with Baby Oil and iodine. At seventy years old, I am paying for every minute I bathed in the sun. Maine Laser Skin Care has helped me literally turn back the hands of time! They are so adept with Botox and fillers but also stay cutting edge treatments such as RF micro-needling,a procedure that has rejuvenated my skin beyond my expectations. Like
Edith H. | QUINCY, MA
★★★★★
I had a great experience at Maine Laser Skin Care! I went to see what the recommendation might be to help soften the wrinkles that had developed in my cheek area. Not only did Chris have a solution she was able to take care of it at the time of my visit! She injected Juvederm in both my upper and lower cheek area and the wrinkles essentially disappeared! I was surprised at what little discomfort I experienced and found Chris to be meticulous and almost as thrilled with my results as I was! Tori, the esthetician, recommended some SkinCeuticals skin care products for my aging skin (I'm 65) that I absolutely love. After just one week of use my skin feels softer and has better color. If you're looking for a facial "tune-up", this is the place to go!
I had a great experience at Maine Laser Skin Care! I went to see what the recommendation might be to help soften the wrinkles that had developed in my cheek area. Not only did Chris have a solution she was able to take care of it at the time of my visit! She injected Juvederm in both my upper and lower cheek area and the wrinkles essentially disappeared! I was surprised at what little discomfort I experienced and found Chris to be meticulous and almost as thrilled with my results as I was! Tori, the esthetician, recommended some SkinCeuticals skin care products for my aging skin (I'm 65) that I absolutely love. After just one week of use my skin feels softer and has better color. If you're looking for a facial "tune-up", this is the place to go!
Karen W. | SYDNEY, ME
★★★★★
I love Maine Laser Skin Care because they are so knowledgeable. You are getting cutting edge services in a caring home town setting!
I love Maine Laser Skin Care because they are so knowledgeable. You are getting cutting edge services in a caring home town setting!
Theresa C. | MILTON, ME
Maine's most trusted esthetician for Botox treatments, laser hair removal, Fraxel skin resurfacing, dermal Juvederm fillers and Latisse.
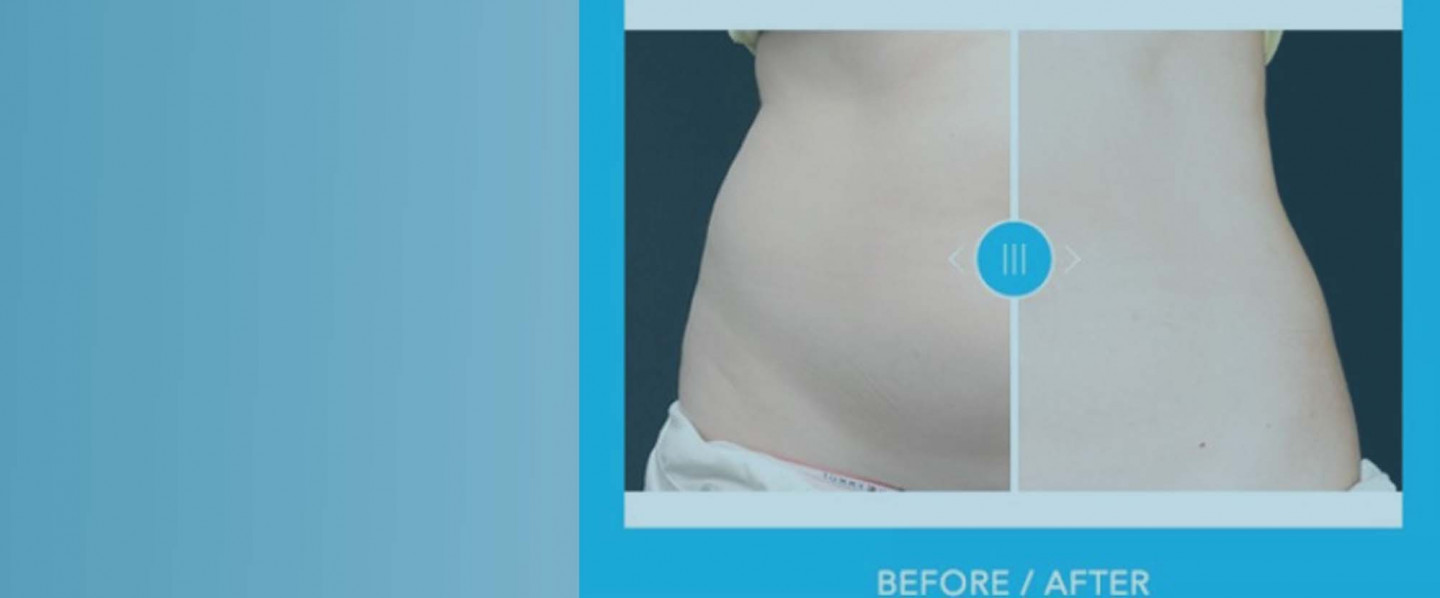
Maine Laser Skin Care has successfully helped thousands of patients throughout Maine, from Augusta and Waterville to Lewiston and Auburn, overcome skin problems ranging from acne to unsightly body hair with cosmetic services. Trust Dr. Burke to provide expert laser treatments and products such as Botox and Juvederm, that will have you feeling confident and looking great. Our skin treatments can take years off of your appearance, helping you to look and feel your best.
Our services can help reduce and remove unwanted hair strands, acne, wrinkles, sun damage, rosacea, broken blood vessels & red and blue facial or leg veins. Experience counts, and Dr. Burke is the most experienced esthetician in central Maine, introducing new technologies as they become available.
John Burke MD is a cum laude graduate of Dartmouth College majoring in Biology, and an honors graduate of Boston University School of Medicine. Dr Burke trained for three years in Internal Medicine at Emory University Affiliated Hospitals in Atlanta, Georgia and is board certified in Internal Medicine. He has practiced as an esthetician for the past 25 years in central Maine and is the founding partner of MidMaine Internal Medicine.
Serving the following areas in Maine:
Albion | Alfred | Auburn | Augusta | Bar Harbor | Bath | Brunswick | Buxton Botox | Buxton Medical Spa | China | Cape Elizabeth | Cape Neddick | Cumberland | Ellsworth | Falmouth | Farmington | Freedom | Freeport | Fairfield | Gardiner | Gorham | Green Hallowell | Harpswell | Kennebunk | Kennebunkport | Kittery | Leeds | Lewiston | Lisbon | Madison | Manchester | New Harbor | Newport | Old Orchard Beach | Oquossoc | Oakland | Pittsfield | Portland | Pownal | Presque Isle | Rockport | Sanford | Saco | Scarborough | Skowhegan | South China | South Portland | Tenants Harbor | Unity | Waterville | Wells | Westbrook Windham Winslow | Wiscasset | Winthrop | Yarmouth | York